

To obtain aluminium, one starts with bauxite ore from which an aluminium oxide, alumina, is extracted through a chemical process.
Bauxite contains 40-60% hydrated aluminium oxide mixed with silica and iron oxide. It is the latter that gives bauxite its characteristic red colour.
The name "bauxite" comes from the village of Les Baux-de-Provence in Bouches-du-Rhône (France), where the mineralogist Pierre Berthier first analysed the ore in 1821.

4 tons of bauxite are needed to obtain 2 tons of alumina, from which 1 ton of aluminium is extracted by electrolysis.
Features :
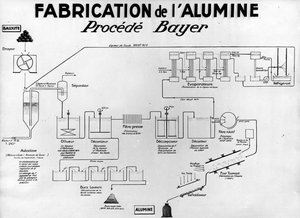
The industrial production process for alumina from bauxite was developed in 1887 by the chemist Karl-Josef Bayer (portrait opposite). It is still used today thanks to important improvements made over time.
The bauxite is crushed then mixed with soda at high temperature and under pressure. The solution obtained, sodium aluminate, is stripped of its impurities, then diluted and cooled, which causes the precipitation of hydrated aluminium oxide. The product of this process is then reduced to a powder to obtain the alumina intended for the production of aluminium.
Discover how we get from alumina to aluminium